Nanodiamond excels as industrial catalyst
Post Date: 30 Mar 2011 Viewed: 645
Nanodiamond could revolutionize the chemical industry by catalysing a reaction used to produce styrene – one of the top 10 biggest chemical industrial processes in the world. So say scientists in China and Europe who have studied the catalytic activity of the nanomaterial. The team found that nanodiamond is nearly three times better than conventional potassium-iron for dehydrogenating ethylbenzene.
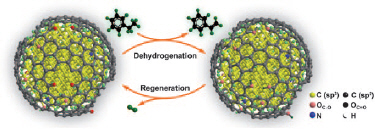
The search for more efficient catalysts is on as engineers try to improve conventional reactions employed in the chemical industry, in an effort to make them less polluting for the environment. Styrene is an important raw material used to synthesize polymers and is generally produced by dehydrogenating ethylbenzene. However, carbon-rich species (coke) are inevitably generated during this process and these materials deposit on the potassium-iron catalysts employed, so greatly deteriorating their activity. Steam must therefore be used to remove the coke, an additional processing step that adds to the overall cost of the reaction.
Although alternatives to iron catalysts have been proposed (such as side-chain alkylation of toluene with methanol and dehydrogenation using oxygen), these methods are either not selective enough or produce flammable mixtures that are difficult to handle. Now Dang Sheng Su of the Shenyang National Laboratory for Materials Sciences at the Chinese Academy of Sciences and co-workers in Germany and Croatia may have come up with another solution to the problem: the researchers propose using nanocrystalline diamond instead when fabricating styrene.
Reactive nanodiamond
There has been a flurry of interest in nanodiamond over the last 10 years or so and it is currently used as a polishing material, polymer additive and lubricant among other things. Synthetic nanodiamonds are between around 4 and 8 nm in size and their large surface-to-volume ratio means that they are more reactive than other forms of carbon, such as carbon nanotubes, nanographite and activated carbon.
The nanodiamond employed by Su and colleagues is commercially produced using detonation synthesis and is rich is sp3 carbon atoms with sp2 graphitic carbon atoms on the surface. These structures were confirmed by ELNES (electron energy-loss near edge structure) measurements.
The researchers analysed nanodiamond's catalytic activity at 550 °C under atmospheric pressure using diluted ethylbenzene as the reactant. They found that the nanomaterial was 2.8 times more active than a potassium-iron catalyst under the same conditions. And that is not all: Su's team found that the high initial activity of the nanodiamond could be fully restored by flowing air through it at 400 °C. The catalyst remained as good as it was at the beginning even after five successive regeneration runs lasting for a total of 120 hours.
According to the researchers, the increased activity comes thanks to the hybrid structure of nanodiamond (the sp3 carbon core covered with graphitic sp2 carbon). "This gives the system a special surface chemistry, which can be put to good use in the dehydrogenation reaction," Su told nanotechweb.org.
"Our nanodiamond catalysts allows for a method that is steam-free, metal-free and coke-free," he added. "While the economic cost still needs to be evaluated, this work is valuable in the search for next-generation industrial catalysts."
The team, which includes researchers from the Ridjer Boskovic Institute Zagreb in Coatia and the Fritz Haber Institute of the Max Planck Society in Germany, reported the work in Angew. Chem. Int. Ed..



